
With Immense pleasure Biomarkers Congress-2019 invites all the participants across the globe to attend the “International Conference on Biomarkers and Clinical Research” during July 15-16, 2019 at Abu Dhabi, UAE. Biomarkers Congress-2019 will uncover different facets of Biomarker study from research to therapy and its applications in divergent fields of Clinical Sciences.
This conference aims to bring together leading academic scientists, researchers and research scholars to exchange and share their experiences and research results on all aspects of Biomarkers and Clinical Research. Biomarkers have the extra potential to identify individuals susceptible to particular diseases. Biomarkers Congress-2019, in the hands of clinical investigators, provide a dynamic and powerful approach to understanding the spectrum of diseases with obvious applications in analytic epidemiology, biomarkers and clinical research in disease prevention, diagnosis and disease management. The cancer biomarker conferences are very much invigorated to extend the knowledge of continuous exposes and scientific research updates in these days. All advances can be evidenced in this biomarker congress.
International Conference on Biomarkers and Clinical Research conference will be organized by ME Conferences which is comprised of 3000+ international Events with over 2000+ Conferences, 1200+ Symposiums and Workshops on multiple fields of clinical, medical, Pharmaceutical, Engineering, Science, Technology, and Business..
We look forward to an exciting 2-day scientific event, business meeting, trade fair and exhibition in the alluring city of Abu Dhabi, UAE
Why attend???
- To ease opportunities for networking, collaboration and exchange of ideas with globally distinguished leaders in Biomarkers research.
- To categorize research and practice-based innovations in optimizing Clinical research and Biomarkers development.
- To discuss and deliberation the challenges and prospects in the new era of optimizing Clinical research and development reforms.
- To dispute gaps and priorities for sustainable development in optimizing Biomarkers research and development.
- To recognize opportunities for evidence-based practice in optimizing Biomarkers research and development.
Who Can Attend?
Oncologists, Radiologists, Hematologists, Immunologists, Neurologists, Cardiologists, Physicians, Geneticists, Psychiatrists, Pathologists, Nurses, Medical Students, Clinical Researchers, Cancer Researchers, Molecular Biologists, Nutritionists, Young Researchers, Bioinformaticians, Medical Devices Specialist, Medical Faculty, Business Entrepreneurs, Data Management Professionals, Pharma Experts, Diagnostics Experts
Sessions/Tracks
Track: Biomarkers
Biomarker is a key molecular or cellular event that links a specific environmental exposure to a health outcome. Biomarkers can be everything that can be correctly measured as an indicator of normal biological functioning, pathogenic paths or a drug response to a therapeutic drug intervention. Biomarkers play a vital role in understanding the relationships between exposure to environmental chemicals, the development of chronic human diseases, and the identification of subgroups that are at increased risk for the disease. Biomarkers are used in many specific fields.
- Medicine
- Regulatory validation
- Cell biology
- Geology and astrobiology
- Ecotoxicology
Track: Clinical Biomarkers
Biomarkers which are used for clinical resolutions are called clinical biomarkers. Clinical biomarkers along with providing an effervescent and significant methodology to understanding the range of many diseases with uses in judgmental and analytic epidemiology, arbitrary clinical trials, screening for drugs or disease diagnosis and prognosis. Well-defined as changes in the constituents of cells or body fluids, these clinical biomarkers offer the means for standardized classification of a disease and risk factors, and the can spread the base information about the underlying pathogenesis of a disease. The main studies in clinical biomarkers are done in the fields of oncology, drug discovery, disease diagnostics and pharmacogenomics.
Track: Advance Biomarker Research and Testing
Biomarker testing is a collection of tests that look for the molecular signs of health so that doctors can plan the best care. Biomarker testing can also be called genetic testing or biomarkers testing. Biomarkers, a foundation of precision oncology, are very important in the management of breast cancer, gastro-esophageal cancer and non-small cell lung cancer.
The major goal of Advances in Biomarker Sciences and Equipment is to accelerate the clinical application of biomarker-based methods to address important challenges in screening, diagnosis, and disease management.
The advances in biomarker testing are mainly in the fields of genetic testing, cancers and other diseases in their diagnosis, treatment and imaging. Testing practices are intensely deliberated, influencing the diagnostic quality and affecting pathologists, oncologists and patients.
Track: Cardiac Biomarkers
Cardiac markers are measured biomarkers to assess cardiac function. They are often discussed in the context of myocardial infarction, but other conditions can lead to an elevation in the level of the cardiac marker. Cardiac biomarkers are elements that are released into the blood when the heart is damaged or strained. A cardiac marker is used in the identification and risk stratification of patients with chest pain and suspected acute coronary syndrome(ACS). These markers include enzymes, hormones and proteins. Cardiac biomarkers have evolved as essential tools in cardiology in the last 50 years, that is, for primary and secondary prevention, the diagnosis and treatment of acute myocardial infarction (AMI), and the diagnosis and stratification of the risk of heart failure.
Track: Genomic Biomarkers
Current genomics and biotechnology promise the development of biomarkers to a state to predict the risk of individual disease that allows early detection of the disease and improves diagnostic classification to better inform individualized treatment. Biomarkers are biological measurements that can be used to predict the risk of diseases, to allow early detection of diseases, to improve the selection of treatment and to monitor the outcome of therapeutic interventions. The main objective of the Human Genome Project was the identification and development of such biomarkers for "personalized, preventive and predictive medicine".
Track: Neurological Biomarkers
Neurological biomarkers are present in the CSF (cerebrospinal fluid), but only at or imperceptibly in the blood. The brain is carefully protected by the blood-brain barrier, which protects it from harmful elements that flow into the bloodstream. Unfortunately for diagnostic purposes, this barricade has also made brain chemistry unattainable for proper blood testing. Neurological biomarkers can be studied using CSF, but this requires an invasive and painful lumbar puncture technique.
Track: Biomarkers in Drug Discovery & Development
Biomarkers have various uses in pharmaceutical R & D. With the current introduction of high-performance instrumentation, protein and gene arrays and bioinformatics, clinical decisions like Drug development and choosing the type of treatment can be made efficiently. Still, there is a deficiency of effective biomarkers to increase the drug development from pre-clinical through all levels of clinical studies. Biomarker in Drug Discovery can also lead to the growth of Modified Medicines.
Track: Immunological Biomarkers
Biomarkers that can control various diseases of the immune system such as autoimmunity, immunodeficiency,cancer, allergies and infections are called immunological biomarkers. As the immune system interacts with all other systems in the body, it plays a very important role in the discovery of biomarkers. Immunological biomarkers, such as miRNA and serum Biomarkers can provide information about the immune response of a body under normal or abnormal conditions.
Track: Biomarkers in Pharmacology
Biomarkers are important tools in drug development and pharmaceutical research. Biomarkers have been recognized by making the drug development process more efficient and have become an essential part of a pharmaceutical and pharmacological research. It is expected that the number of biomarkers will ultimately transition into the clinical laboratory.
The biomarkers demonstrate the dose-response relationships of the drug between changes in biomarkers, the onset of the injury and the severity of the injury corresponding to the progression of the injury.
Track: Biomarkers in Oncology
Biomarkers play an important role in oncology that is used in clinical research for risk assessment, differential diagnosis, determination of the nature of tumors, for detection purposes, to determine the prognosis and dose-response of the drug to the treatment. The process indicative of the presence of cancer in the body is carried out by means of tools known as cancer biomarkers. There are different types of cancer in which biomarkers have their application, such as lung, melanoma, breast, colorectal, pancreas and many more. Biochemically, cancer biomarkers can be DNA, RNA, proteins, peptides, hormones, metabolites and even biological processes such as apoptosis, angiogenesis or proliferation. There are three main types of cancer biomarkers depending on their clinical use: prognostic, predictive and pharmacodynamic markers.
Track: Clinical Research
Clinical research is a branch of social security science that decides the well-being and adequacy (viability) of the solutions, devices, analytical elements and treatment regimes foreseen for human use. These could be used for counterproductive action, treatment, findings or to calm the side effects of some diseases. Clinical research is exclusive in relation to clinical practice. Accumulated medications are used in clinical practice, while in clinical research confirmation is gathered to set up a treatment.
Track: Clinical Trials
Clinical trials are tests performed in clinical research. Such imminent thoughts of biomedical or behavioral research in human members are intended to answer particular queries regarding biomedical or behavioral mediations, including new medications (eg. new antibodies, medications, dietary decisions, dietary supplements and medical devices) and intercessions. Known that require studies and exams. Clinical trials produce information on safety and viability.
Market Analysis Report
Global Market Analysis
The market for biomarker technologies is assigned to reach USD 74.51 billion for 2022 from USD 46.97 billion in 2017, at a compound annual rate of 9.7% during the expected time frame.
Dependent on the type of product, the market for biomarker technologies is separated into devices, amenities information technology. In 2018, the consumables part depended on to signify the largest market share due to its higher sales volume compared to the instruments. The consumables marketplace is further disjointed into kits, reagents and chromatography columns. In 2018, the sets and reagents segments denoted the largest in the consumables market.
Although critical improvements have been made in the area of biomarkers, deducing them priceless clinical trials is dejected and a major problem to the clinical use of these biomarkers. The advance of this market is obsessed by factors such as the growth of health spending, the increase in R & D funding, and the increase in the usefulness of biomarkers for investigative purposes and the growth in the research of biomarkers.
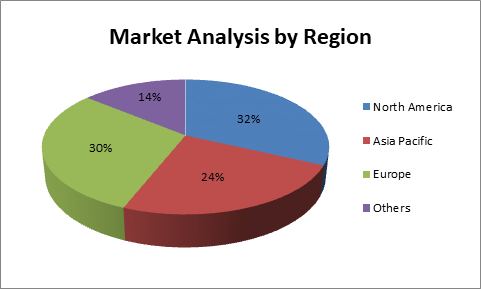
In 2017, North America signified the major share in the biomarker market. The Asia Pacific verified the highest growth from 2017 to 2022 for the biomarker advances to market. Factors such as the growing rate of malignancy, the development of the geriatric population and the support of private and public links for biomarker research are driving the development of this regional section. By influencing advance, the biomarker breakthrough market is categorized by immunoassays, PCR, mass spectrometry, NGS, chromatography, cytogenetic and other different advances. The immunoassay percentage accounted for most of it in 2017. This section is additionally categorized into ELISA, western blot and protein microarrays. In 2017, the ELISA fragment exemplified the major share in the immunoassay market.
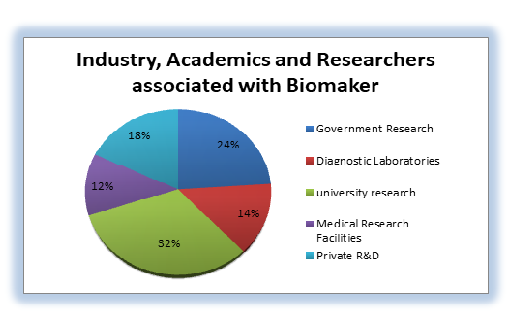
Statistical analysis of members associated with Biomarkers
Market Analysis of Biomarkers by Region
Scope and Importance
The biomarker market presents the highest of all prospects in sub-segments of representative diseases. This study examines one of the most important engines of the market: the overview of combination analyses, which are estimated to continue contributing significantly to market development during the forecast period. This report studies emerging markets by disease segment. Endless growth is estimated in emerging geographies, driven by the growing middle class in emerging countries such as India, China, Brazil, and Russia. This report seeks to address issues of critical importance to analyze a changing market dynamics, emerging players and machinery, approaches to access emerging markets and specific disease segments and geographies to allocate resources and make operative decisions. For companies with an actual approach, the market opportunity expects you. It is important to emphasize that the ability to develop an effective strategy begins with the place where the opportunity exists and ends with the way to execute effectively to obtain the opportunity's profits.
Why Abu Dhabi?
Biomarkers Congress-2019 will be held in Abu Dhabi. Abu Dhabi has begun as a global city and a Middle East business midpoint. Abu Dhabi ranked 44th between the best financial cities in the world and the 27th richest city in the world in 2012. It is also a global economic epicenter and ranks 37th among the 50 most important financial cities in the world and the first in the Middle East. According to a study report on the future effectiveness of cities, by 2024, Abu Dhabi will have increased to 23rd in the index. Cancer research is being accompanied mainly in Asian countries near Abu Dhabi, making it a suitable destination for oncology and cancer conferences.
Biomarkers Technologies Market, By Research Area:
-
Proteomics
-
Genomics
-
Metabolomics
-
Lipidomics
-
Other research types
Major associations around the world
Biomarkers society
-
The Biomarker consortium
-
International society for oncology and Biomarkers
-
Biomarkers profile corporation
-
World molecular imaging society
-
European society of molecular imaging
-
Society of nuclear medicine and molecular imaging
Universities associated with Biomarkers
Top Universities in UAE
-
Dubai Medical College for Girls
-
Gulf Medical University Ajman
-
United Arab Emirates University
-
University of Sharjah
-
Ras al-Khaimah Medical and Health Sciences University
Related Conferences:
International Conference on Molecular Markers and Cancer Therapeutics, January 25-26, 2019 Dubai, UAE
12th International Conference on Pharmacoepidemiology and Clinical Research, March 18-19, 2019 Dubai, UAE
3rd World Congress on Radiology and Oncology, April 08-09, 2019 Abu Dhabi, UAE
International Conference on Biomarkers and Cancer Targets, November 18-19, 2019 Dubai, UAE
2nd Annual Biomarkers Congress, December 06-07, 2019 Berlin, Germany
ISOBM (International Society of Oncology and BioMarkers) 2018 Congress, 24 - 27 November 2018 Hamburg, Germany
2nd International Conference on Cancer Biology, Therapeutics and Drug Discovery & Delivery October 03-04, 2018 Los Angeles, California, USA
2nd Global Meeting on Clinical Oncology and Radiology March 27-28, 2019 Hong Kong
Circulating Biomarkers World Congress 2019, March 27-29, 2019, Coronado Island, California
Related Societies:
Societies in USA:
ACRP: Association of Clinical Research Professionals | American Association for Cancer Research | American Cancer Society | American Cancer Society | American Cancer Society Cancer Action Network | American Federation for Medical Research: AFMR | American Society of Clinical Oncology | American Society of Clinical Oncology | ASCO | Anxiety and Depression Association of America, ADAA | Association of Clinical Research Organizations | Association-Clinical Research | CGD Society - Clinical trials | Clinical Research Association of Canada | Clinical Trials - Alzheimer's Association | Clinical Trials - MPS Society | Clinical Trials – Society for Women's Health Research | American Thyroid Association | Clinical Trials | Leukemia and Lymphoma Societ | Clinical Trials | National CML Society | Clinical Trials Veterinary Cancer Society | Clinical Trials Network | Clinical Trials: American Diabetes Association | CRS : Clinical Research Society | DIA - Clinical Research - Drug Information Association | Dysphagia Research Society | Dystonia Medical Research Foundation | DMRF | International Association of Clinical Research Nurses | National Brain Tumor Society | Osteoarthritis Research Society International (OARSI) | Society for Clinical Data Management (SCDM) | Society for Clinical Trials (SCT) | SOCRA The Society of Clinical Research Associates | T21RS | The American Society of Hematology | USA & Canada - Society of Clinical Research Associates | American Association for Cancer Research | American Breast Cancer Foundation
Societies in Europe:
The European Association for Clinical Pharmacology and Therapeutics (EACPT) | Academy of Physicians in Clinical Research | Association of European Cancer Leagues (ECL) | Association of Medical Research Charities: AMRC | Breast International Group (BIG) | Cardiovascular and Interventional Radiological Society of Europe (CIRSE) | DIA - Clinical Research - Drug Information Association | EACR - European Association for Cancer Research | ECFS Clinical Trials Network | ECRIN: Facilitating European Clinical Research | ENGOT | ESA Clinical Trial Network | ESCMID | ESDR: European Society for Dermatological Research | ESMO: European Society for Medical Oncology | ESPR European Society for Paediatric Research | European Association for Cancer Research (EACR) | European Association of Neuro-Oncology (EANO) | European Association of Nuclear Medicine (EANM) |European Association of Urology (EAU) | European CanCer Organisation: ECCO | European Oncology Nursing Society (EONS) | European Organisation for Research and Treatment of Cancer (EORTC) | European School of Oncology (ESO) | European Sleep Research Society | European Society for Clinical Investigation
Societies in Middle East:
Moazzara – Emirates Association for Cancer Support| Angels of Mercy Cancer Society | Friends of Cancer Patients Society | Association for Molecular Pathology| Middle-Eastern Association for Cancer Research (MEACR) | Iranian Cancer Association (ICA) |Iranian Society of Medical Oncology and Hematology (ISMOH)| Indonesian Association of Clinical Pathology and Laboratory Medicine |Romanian Society of Biochemistry and Molecular Biology |Romanian Society of Human Genomics | The Malaysian Biochemical Society| Iranian Society of Clinical Oncology (ISCO) | Pakistan Society of Clinical Oncology (PSCO)
Societies in Asia:
Association of Oncologists of Uzbekistan | Chinese Society of Clinical Oncology (CSCO) | Hong Kong Cancer Therapy Society | Indian society clinical research | Indian Society of Medical & Paediatric Oncology (ISMPO) | Indonesian Society of Hematology and Medical Oncology (ISHMO) |Japan Society of Clinical Oncology (JSCO) | Japanese Society of Medical Oncology (JSMO) | Korean Association for Clinical Oncology (KACO) | Malaysian Oncological Society | Medical Oncology Group of Australia (MOGA) | Myanmar Oncology Society | New Zealand Association of Clinical Research | New Zealand Society for Oncology (NZSO) | Oncology Club, Bangladesh | Philippine Society of Medical Oncology (PSMO) | Singapore Society of Oncology (SSO) | Taiwan Oncology Society (TOS) | Thai Society of Clinical Oncology (TSCO)
Past Conference Report
Biomarkers Congress 2017
Epigenetics and Biomarkers 2017: Past Conference Report
The success of the Epigenetics and Biomarkers 2017 conference has given us the prospect to bring the gathering one more time. Conferenceseries LLC hosted the “International Conference on Cancer Epigenetics and Biomarkers” during October 26-28, 2017 at Hyatt Regency Osaka, Japan.
The conference was focused on learning about Oncologists with the theme “From Novel therapies to Revolutionary Strategies, Diagnosis and Treatment”. The conference mainly directs towards addressing main issues like Prevention, Diagnosis, and Treatment diseases of the Organ-related Cancers and its innovative techniques. Epigenetics and Biomarkers 2017 is one of the most appreciated Cancer Conferences organized by Conferenceseries LLC. This Oncologists Meeting was discussed on methods and strategies related to management, quality improvement of Cancer as well as to explore the new ideas and concepts on global scale and the topics include lung cancer, breast cancer, bone cancer, blood cancer, cervical cancer, colon-rectum cancer, prostate cancer, thyroid cancer.
The conference was embarked with an opening ceremony followed by Keynote sessions and followed by series of lectures delivered by both Honorable Guests and members of the Keynote forum. The adepts who promulgated the theme with their exquisite talk were:
Dr. Suraiya Rasheed, University of Southern California, United States
Dr. Jenny Wang, University of New South Wales, Australia
Dr. Manal H. Al Khanbashi, Higher College of Technology, Oman
Dr. Pedro Sanchez Rovira, Xanit International Hospital, Spain
Mr. David A Ligon, Oncology Fox, United States
Dr. Atsushi Kaneda, Chiba University, Japan
Dr. Glenn Deng, Stanford University School of Medicine, United States
Dr. Ladan Teimoori-Toolabi, Molecular Medicine Department, Pasteur Institute of Iran, Iran
Dr. Sang Hyun Lee, Duke-NUS Medical School, Singapore
Dr. Jose Perez del Palacio, MEDINA Foundation, Spain
Dr. Safana Salim Al Saidi, Ministry Of Health, Muscat
Dr. In-Hye Jung, University of Ulsan College of Medicine, Seoul, South Korea
Conferencesereies LLC offers its heartfelt appreciation to Organizing Committee Members, dexterous of field, various outside experts, company representatives and is obliged to other eminent personalities who interlaced with Conferencesereies LLC and supported the conference in every aspect, without which the conference would not have been possible.
We would like to announce the commencement of the International Conference on Biomarkers and Clinical Research during July 15-16, 2019 at Abu Dhabi, UAE.
We welcome all the eminent researchers, students and delegate participants to take part in this upcoming conference to witness invaluable scientific discussions and contribute to future innovations in the field of Oncology and Cancer Research.
For More details: https://biomarkerscongress.conferenceseries.com/
Let us meet again @ BIOMARKERS CONGRESS-2019
